By Category
Blog Categories
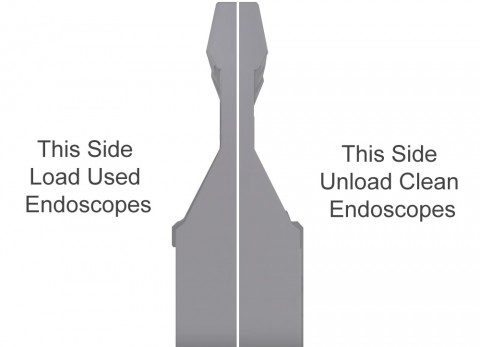
One-way AER workflow
On April 18, 2018 there was big news for Cantel Medical as the FDA issued 510(k) clearance for them to begin marketing their Advantage Plus Pass-Thru automated endoscope reprocessor (AER). According to their press release this would be the first pass-through reprocessor marketed in the United States.
The Cantel design of the Advantage Plus Pass-Thru AER enables one-way workflow. This dramatically reduces the possibility of human error. It's proven to high-level disinfect commonly used endoscopes and duoscopes. The design includes hard-wall separation. This allows for a completely separate area, or room for that matter, one for decontamination on one side and the extraction of the decontaminized endoscopes and storage of them in the second area or room. This physical separation prevents cross contamination or re-contamination with virtually hands-free operation with an automatic cycle start for ease of operation. Reducing the amount of handling of the endoscopes decreases the potential recontamination of reprocessed endoscopes.
Separation technology implemented is still considered globally to be one of the best-practices supporting infection prevention objectives and guidelines.
Healthcare-associated infections (HAIs) are a common issue in healthcare facilities. Patients who contract these HAIs can experience life changing consequences or even death. So setting up strict guidelines and purchasing and implementing technology that reduce these risks just makes good sense for doctor's offices across the nation.
- 1.7 million people contract an HAI every year i
- 99,000 people die from an HAI each year i
- 1 in 25 hospital patients may contract an HAI. i
And considering that more HAIs have historically been linked to endoscope contamination ii than to all other medical devices the investment in better and safer disinfecting technologies for endoscopes is something that is critically important and should not be put off.
This new model allows the reprocessing of 4-5 endoscopes in just a little over 1 hour. It also uses Cantel's RAPICIDE® PA Disinfectant.
i Centers for Disease Control, Preventing Healthcare-Associated Infections. Retrieved from CDC: https://www.cdc.gov/washington/~cdcatWork/pdf/infections.pdf
ii Rutala, W.A., Weber, D. J., and the Healthcare Infection Control Practices Advisory Committee (2008). Guideline for Disinfection and Sterilization in Healthcare Facilities (Last update: February 15, 2017). https://www.cdc.gov/infectioncontrol/pdf/guidelines/disinfection-guidelines.pdf